The Difficulty of Direct Electrolysis of Seawater for Hydrogen Production Has Finally been Solved
From:
Zhonglin International Group Date:12-01 1318 Belong to:Industry Related
On November 30, 2022, Academician Xie Heping and his doctoral team from Shenzhen University/Sichuan University, with Shenzhen University as the first unit, published their research findings on Nature titled "A membrane based seawater electrolyte for hydrogen generation". This study, for the first time, combines physical mechanics and electrochemistry to establish a new principle and technology for in-situ direct electrolysis of seawater without desalination driven by phase change migration. It takes a new approach and completely isolates seawater ions, achieving a breakthrough in efficient in-situ direct electrolysis of seawater without desalination process, side reactions, and external energy consumption (i.e. using seawater as pure water and directly electrolyzing hydrogen in situ in seawater), Breaking through the half century long problem of direct electrolysis of seawater for hydrogen production, it is expected to form China's original "marine green hydrogen" global emerging strategic industry.
The Nature review expert commented on the achievement: "Few papers can convincingly achieve large-scale and stable hydrogen production from seawater, but the work of this paper precisely achieves this. They perfectly solve the problem of harmful corrosiveness that has long plagued the field of seawater hydrogen production, will open the door to low-cost fuel production, and have the potential to drive change towards a more sustainable world!"

The principle of in-situ direct electrolysis of seawater without desalination driven by phase change migration for hydrogen production
Green hydrogen energy is an important direction for future energy development. With the explosive growth of hydrogen energy, it is expected that by 2060, the annual demand for hydrogen in China will reach 130 million tons, requiring approximately 1.17 billion tons of pure water for electrolysis each year. However, the scarcity of freshwater resources will seriously constrain the development of "green hydrogen" technology. The ocean is the largest hydrogen mine on Earth, and seeking water from the ocean is an important direction for the future development of hydrogen energy. However, the complex composition of seawater (about 92 chemical elements) poses many challenges and challenges for hydrogen production from seawater. Desalination followed by hydrogen production is currently the most mature seawater hydrogen production technology path, and large-scale demonstration projects have been carried out in multiple countries around the world. However, this type of technology heavily relies on large-scale desalination equipment, with complex process flow and occupying a large amount of land resources, further increasing the cost of hydrogen production and the difficulty of engineering construction. In the early 1970s, scientists proposed that seawater could be directly electrolyzed to produce hydrogen? Over the past half century, well-known research teams at home and abroad, such as Stanford University in the United States, the French National Science Research Center, the University of Adelaide in Australia, the Chinese Academy of Sciences, have carried out a lot of exploration and research through catalyst engineering, membrane material science and other means, aiming to solve the problems of chlorine evolution side reaction, calcium magnesium precipitation, catalyst deactivation and other problems faced by the direct electrolysis of seawater for hydrogen production. However, there has never been a breakthrough theory and principle that completely avoids the impact of complex components of seawater on electrolytic hydrogen production. The principle and technology of large-scale, efficient, and stable direct electrolysis of seawater for hydrogen production are still blank in the world.
Academician Xie Heping proposed a new approach that combines physical mechanics and electrochemistry to solve the difficulties and challenges faced by direct electrolysis of seawater for hydrogen production, thus creatively creating a new principle and technology for in-situ electrolysis of seawater without desalination. This achievement cleverly combines physical and mechanical processes such as molecular diffusion and interface phase equilibrium with electrochemical reactions to establish a theoretical model for direct electrolysis of hydrogen in seawater driven by phase transition migration. It reveals the mechanism of the influence of interface pressure difference on spontaneous phase transition mass transfer in seawater under micrometer scale air gap pathways, and forms a dynamic self regulating stable electrolysis hydrogen production method that synergizes electrochemical reactions with seawater migration, Solved the half century long problem of harmful corrosiveness in the field of seawater electrolysis hydrogen production (applied patents: CN2021110197054; CN114481164A; CN2022110704439; CN2022110748884; PCT/CN2022/128225). At the same time, Academician Xie Heping's team developed the world's first 400L/h in-situ direct electrolysis hydrogen production technology and equipment, which ran continuously in the seawater of Shenzhen Bay for over 3200 hours, convincingly achieving stable and large-scale hydrogen production from seawater. In addition, the research team further developed acidic and alkaline solid gel electrolytes to show that the phase change migration strategy is suitable for different electrolyte materials, and is expected to accompany the iterative development of PEM and AEM electrolysis technology. It is reported that this principle technology can explore and promote the direct in-situ hydrogen production of diversified water resources (such as river water, wastewater, salt lakes, etc.), providing new ideas for resource enrichment and energy production with multiple benefits. As highly praised by the reviewers of Nature for this study.
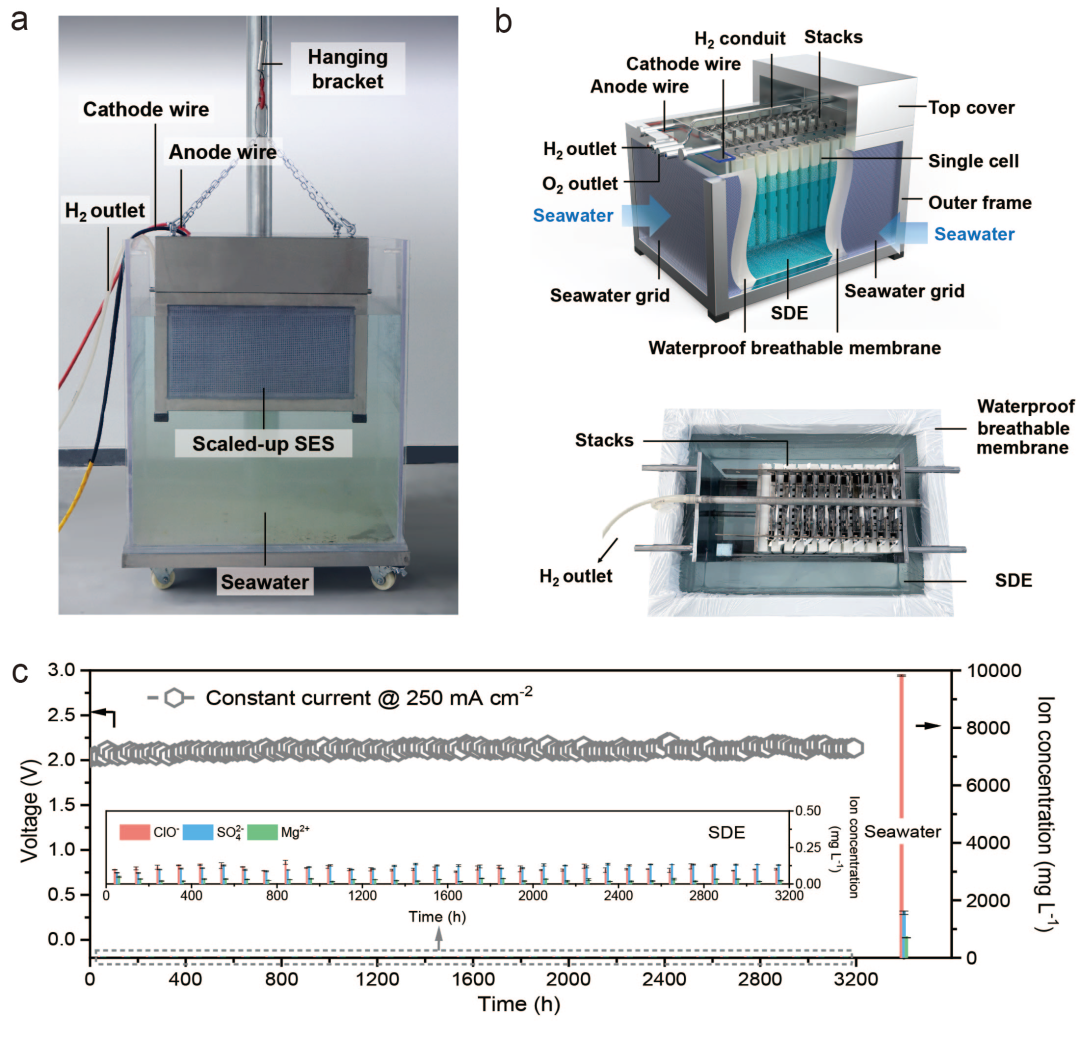
Scaled technical equipment and stability of in-situ direct electrolysis of seawater without desalination for hydrogen production
The integration of multi-disciplinary theory of in-situ electrolysis of hydrogen from seawater without desalination is an original principle and technological breakthrough independently developed in China, opening up a new path for global seawater hydrogen production; This technology can integrate the utilization of renewable energy such as offshore wind power, seawater resource utilization, and hydrogen production. It is expected to form a new in-situ seawater direct electrolysis hydrogen production model without desalination, additional catalyst engineering, seawater transportation, and pollution treatment, truly transforming the inexhaustible "seawater resources" into "seawater energy". This technology can be used in the future to build an integrated in-situ seawater hydrogen production plant that combines with offshore renewable energy, which is expected to become a key breakthrough in the large-scale development of offshore renewable power, accelerating the formation of a multi energy complementary Chinese original "marine green hydrogen" global emerging strategic industry.
The Nature review expert commented on the achievement: "Few papers can convincingly achieve large-scale and stable hydrogen production from seawater, but the work of this paper precisely achieves this. They perfectly solve the problem of harmful corrosiveness that has long plagued the field of seawater hydrogen production, will open the door to low-cost fuel production, and have the potential to drive change towards a more sustainable world!"

The principle of in-situ direct electrolysis of seawater without desalination driven by phase change migration for hydrogen production
Green hydrogen energy is an important direction for future energy development. With the explosive growth of hydrogen energy, it is expected that by 2060, the annual demand for hydrogen in China will reach 130 million tons, requiring approximately 1.17 billion tons of pure water for electrolysis each year. However, the scarcity of freshwater resources will seriously constrain the development of "green hydrogen" technology. The ocean is the largest hydrogen mine on Earth, and seeking water from the ocean is an important direction for the future development of hydrogen energy. However, the complex composition of seawater (about 92 chemical elements) poses many challenges and challenges for hydrogen production from seawater. Desalination followed by hydrogen production is currently the most mature seawater hydrogen production technology path, and large-scale demonstration projects have been carried out in multiple countries around the world. However, this type of technology heavily relies on large-scale desalination equipment, with complex process flow and occupying a large amount of land resources, further increasing the cost of hydrogen production and the difficulty of engineering construction. In the early 1970s, scientists proposed that seawater could be directly electrolyzed to produce hydrogen? Over the past half century, well-known research teams at home and abroad, such as Stanford University in the United States, the French National Science Research Center, the University of Adelaide in Australia, the Chinese Academy of Sciences, have carried out a lot of exploration and research through catalyst engineering, membrane material science and other means, aiming to solve the problems of chlorine evolution side reaction, calcium magnesium precipitation, catalyst deactivation and other problems faced by the direct electrolysis of seawater for hydrogen production. However, there has never been a breakthrough theory and principle that completely avoids the impact of complex components of seawater on electrolytic hydrogen production. The principle and technology of large-scale, efficient, and stable direct electrolysis of seawater for hydrogen production are still blank in the world.
Academician Xie Heping proposed a new approach that combines physical mechanics and electrochemistry to solve the difficulties and challenges faced by direct electrolysis of seawater for hydrogen production, thus creatively creating a new principle and technology for in-situ electrolysis of seawater without desalination. This achievement cleverly combines physical and mechanical processes such as molecular diffusion and interface phase equilibrium with electrochemical reactions to establish a theoretical model for direct electrolysis of hydrogen in seawater driven by phase transition migration. It reveals the mechanism of the influence of interface pressure difference on spontaneous phase transition mass transfer in seawater under micrometer scale air gap pathways, and forms a dynamic self regulating stable electrolysis hydrogen production method that synergizes electrochemical reactions with seawater migration, Solved the half century long problem of harmful corrosiveness in the field of seawater electrolysis hydrogen production (applied patents: CN2021110197054; CN114481164A; CN2022110704439; CN2022110748884; PCT/CN2022/128225). At the same time, Academician Xie Heping's team developed the world's first 400L/h in-situ direct electrolysis hydrogen production technology and equipment, which ran continuously in the seawater of Shenzhen Bay for over 3200 hours, convincingly achieving stable and large-scale hydrogen production from seawater. In addition, the research team further developed acidic and alkaline solid gel electrolytes to show that the phase change migration strategy is suitable for different electrolyte materials, and is expected to accompany the iterative development of PEM and AEM electrolysis technology. It is reported that this principle technology can explore and promote the direct in-situ hydrogen production of diversified water resources (such as river water, wastewater, salt lakes, etc.), providing new ideas for resource enrichment and energy production with multiple benefits. As highly praised by the reviewers of Nature for this study.

Scaled technical equipment and stability of in-situ direct electrolysis of seawater without desalination for hydrogen production
The integration of multi-disciplinary theory of in-situ electrolysis of hydrogen from seawater without desalination is an original principle and technological breakthrough independently developed in China, opening up a new path for global seawater hydrogen production; This technology can integrate the utilization of renewable energy such as offshore wind power, seawater resource utilization, and hydrogen production. It is expected to form a new in-situ seawater direct electrolysis hydrogen production model without desalination, additional catalyst engineering, seawater transportation, and pollution treatment, truly transforming the inexhaustible "seawater resources" into "seawater energy". This technology can be used in the future to build an integrated in-situ seawater hydrogen production plant that combines with offshore renewable energy, which is expected to become a key breakthrough in the large-scale development of offshore renewable power, accelerating the formation of a multi energy complementary Chinese original "marine green hydrogen" global emerging strategic industry.



